Next to the vessels with the odors is another flask containing a green liquid. This liquid has to be mixed with other substances for the next reaction step. To do this, the liquid needs to be added in the same concentration as it is in the flask. However, the remaining amount in the flask is no longer sufficient for the reaction. To solve the problem, you only have a concentrate of the green liquid. Create a dilution series to determine the correct concentration and thereby obtain an important clue!
Equipment needed:
- 5 test tubes
- Test tube holder
- 10 mL measuring cylinder
- Pipettes
- 100 mL beaker filled with water
- 100 mL beaker for waste
- Concentrated dye solution
- Unknown dye sample in a test tube
- Permanent marker
- Notepads, pen, calculator
Experiment instruction:
- Place the 6 test tubes (TT) in the test tube holder and mark them in sequence with the numbers 1 to 5 and a question mark.
- Fill the unknown sample into the test tube labeled with the question mark.
- Measure out 10 mL of the dye concentrate and transfer this to the test tube with the number 1 (TT1).
- Using a pipette and the rinsed graduated cylinder, transfer 4 mL of solution from TT1 to TT2. Note: Pipettes can continue to be used for the experiment after rinsing with water.
- Fill TT2 with 6 mL of water and homogenize the solution by carefully shaking and swirling. Use the rinsed measuring cylinder for this purpose.
- Repeat steps 4 & 5 with the newly prepared solution until you have obtained a dilution series with a total of 5 solutions (photo).
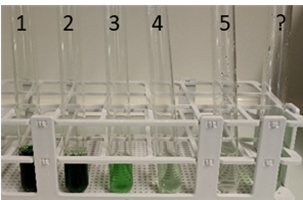
Task:
Determine the concentration of the unknown sample using the dilution series prepared!
- Assign a solution from the dilution series to the unknown sample (TT?). Note: To do this, match the color intensity of the test tubes and assign those with the most similar color impression to each other. Make sure that you observe all test tubes under identical light conditions, with the same background and perspective.
- TT? corresponds approximately:
- Determine the concentration of the unknown solution using the following table and the dilution steps performed. If necessary, use another sheet for secondary calculations.
| Test tube | Concentration |
| TT1 (=concentrated dye) | 250 mg/L |
| TT2 | |
| TT3 | |
| TT4 | |
| TT5 | |
| TT? |
Find the correct number for the code:
The concentration of the dye in the unknown sample has the following concentration:
- Solution A: Concentration = 40 mg/L and code: 3
- Solution B: Concentration = 16 mg/L and code: 2
- Solution C: Concentration = 6,4 mg/L and code: 8




