As you have been informed by some information on the whiteboard, you now have to obtain a solid in a chemical way. Any idea how this will work?
Stick to the experimental procedure and observe carefully!
Equipment needed:
- 4 test tubes with metal salt solutions (prepared by the teacher or you will receive salt solution you have to transfer in the test tubes (around 3 mL needed of each salt solution). Do not forget to label the test tubes).
- 1 M NaOH (~ 10 ml per group)
- Pipettes
Experiment instructions:
- Add about 0.5 mL of 1 M NaOH to each metal salt solution using the pipette.
- For each metal salt, write down your observations on the following criteria:
- Is a precipitate visible after adding 0.5 mL NaOH each time?
- Color of precipitation
- Repeat step 1 four more times and note which metal salts cause the precipitate to dissolve again by adding NaOH several times.
Hint 1:
Concentration gradients can falsify the result. To avoid concentration gradients, the test tubes should be closed with a stopper and shaken after each addition of NaOH.
Hint 2:
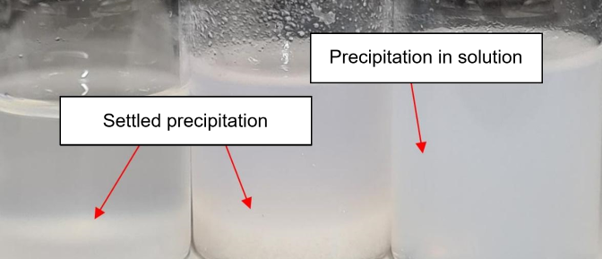
Precipitation is the settling out of a sparingly soluble solid from a solution. However, a fine solid distributed in the solution also counts as precipitate (see figure).
Task:
Compare your observations with the following solution variants. In which of the following variants are all three statements completely correct:
Solution A (code: 3)
- The aluminum salt precipitates blue-green.
- For sodium chloride, no precipitation can be observed at any time.
- The precipitation of the iron salt remains even after multiple additions of NaOH.
Solution B (code: 7)
- The iron salt precipitates grey-green.
- No precipitation can be observed for the aluminum salt at any time.
- The precipitation of the manganese/barium salt* is white
Variante C (Code: 6)
- For sodium chloride, no precipitation can be observed at any time.
- The precipitate of the aluminum salt redissolves after several additions of NaOH.
- The precipitation of the manganese/barium salt* remains even after multiple additions of NaOH.
* You will be given either a manganese or a barium salt, depending on the availability at your school.




